Ethicon / Johnson & Johnson issued an urgent field safety notice in 2016, advising of a worldwide recall of its Ethicon Physiomesh™ Flexible Composite Mesh. Ethicon Physiomesh™ Flexible Composite Mesh is a medical device employed by surgeons in the repair of patients’ hernias.
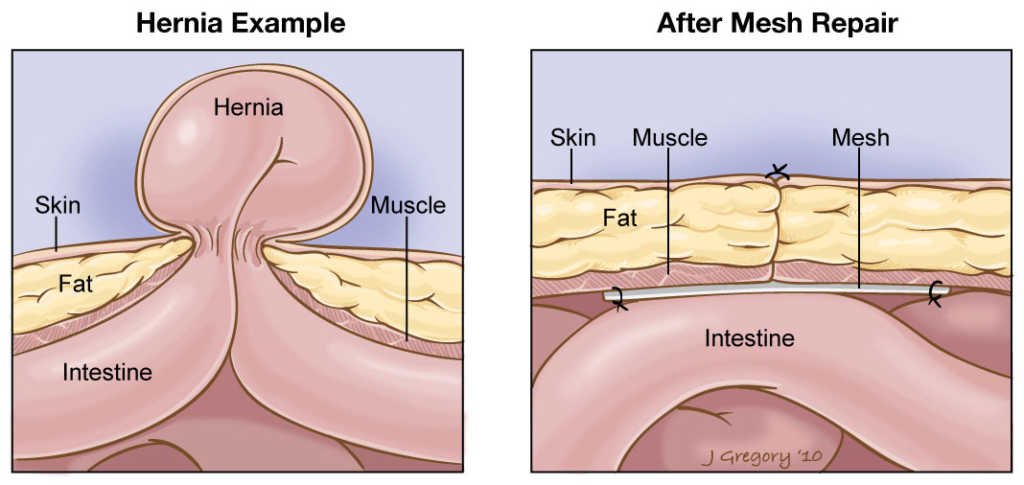
A hernia is a protrusion (bulge) of part of a body cavity (usually an intestine or abdomen) outside of the area in which it belongs. While some hernias may not harm a patient, others require emergency surgery. During this surgery, surgeons often use hernia mesh implants such as the Ethicon Physiomesh™ Flexible Composite Mesh to provide greater stability to weakened tissue.
Unfortunately, many of these devices have been linked to serious injury and wrongful death. Feldman & Pinto’s Philadelphia Hernia Mesh Complications Lawyers are currently evaluating cases involving injuries from Ethicon Physiomesh™ Flexible Composite Mesh and other hernia mesh devices.
Recall of Ethicon Physiomesh™ Flexible Composite Mesh
Sales of Ethicon Physiomesh™ Flexible Composite Mesh began in March 2010, after FDA approval through a 510(k) application. A 510(k) application allows the sale of a product without clinical trials or studies establishing its safety. This sort of approval is limited to products that are “similar” to other products already on the market.
Six years later, Ethicon initiated the global recall of its Ethicon Physiomesh™ Flexible Composite Mesh line after studying unpublished data from two independent hernia registries. The data showed a higher than average rate of returning (recurring or reopening) hernias and repeated hernia operations after use of Ethicon Physiomesh™ Flexible Composite Mesh.
The safety notice lists and describes the particular hernia mesh devices subject to recall. (Although Ethicon / Johnson & Johnson claims that it “voluntarily withdrew” rather than recalled the devices, the safety notice clearly states that Ethicon recalled the products.)
Ethicon Physiomesh™ Flexible Composite Mesh Lawsuits
Patients have filed lawsuits against Ethicon / J&J, alleging that Ethicon Physiomesh™ Flexible Composite Mesh is a dangerous and defective medical device that causes serious personal injuries. These injuries may include:
- bowel obstruction
- hernia mesh migration
- serious and life-threatening infections
- shrinkage / contraction of hernia mesh implants
- tearing of hernia mesh and other mesh device failures
Patients have also alleged that the coated synthetic polypropylene used to make Ethicon Physiomesh™ Flexible Composite Mesh is responsible for many of these dangerous complications. The coating prevents the patient’s abdominal wall from absorbing the mesh. When the abdominal wall cannot absorb the hernia implant, the device can migrate (move around), causing:
- breakage of mesh material, and
- perforation of internal organs
The Philadelphia Hernia Mesh Complications Lawyers at Feldman & Pinto are evaluating cases from patients injured by Ethicon Physiomesh™ Flexible Composite Mesh. If you sustained injuries after surgery employing hernia mesh implants, please contact our Philadelphia Personal Injury Lawyers for a free evaluation of your case. If you suffered an infection from hernia mesh, our Philadelphia Hernia Mesh Infection Lawyers can advise you on your legal options.