NOTE: Feldman & Pinto no longer handles cases related to injuries caused by Watchman devices.
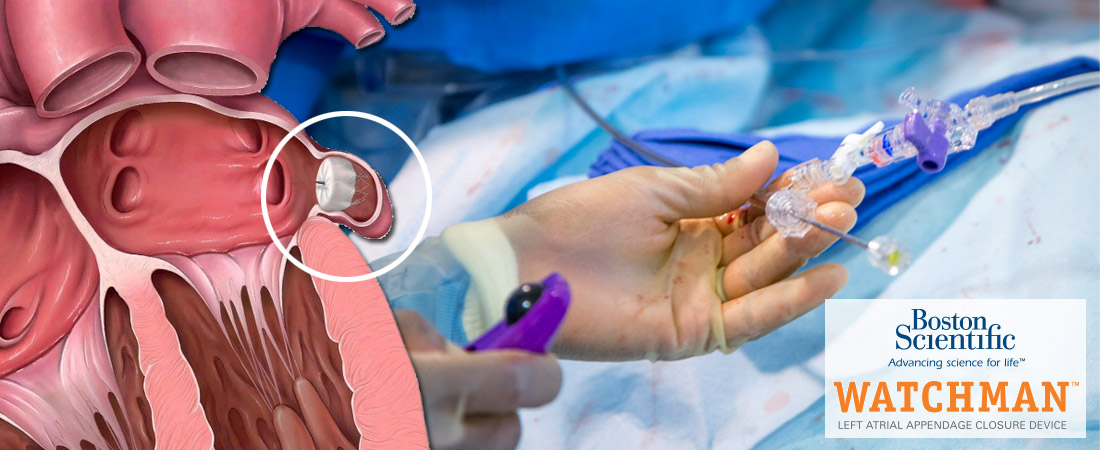
Studies conducted before and after FDA approval of Boston Scientific’s anti-stroke device show that Watchman stroke devices can cause serious injuries both during and after implantation. Alternate names for Watchman stroke devices include:
- Watchman Left Atrial Appendage Closure Device
- Watchman Stroke Prevention Device
- Watchman Anti-stroke Device
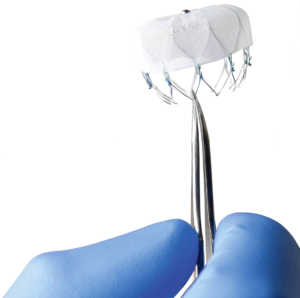
While the device may eliminate a patient’s risk of excessive bleeds from blood thinners, it has problems of its own with equal or greater risks. Studied cases indicate that Watchman stroke devices can cause serious injuries due to:
- improper insertion of the device
- breakage and movement of the device after it has been implanted, and
- blockages in several areas of patients’ hearts
Injuries Caused during Insertion of Watchman Stroke Devices
Watchman stroke devices can cause serious injuries even before the completion of device implantation. This may be due to:
- inadequate insertion instructions provided by the manufacturer, and
- the time and practice required to learn how to safely insert the device
In 2015, Boston Scientific recalled nearly 30,000 of the devices after reports of injuries sustained during the device’s insertion. A 2016 study found a high incidence of injuries during Watchman device insertion.
The manufacturer itself has acknowledged that a learning curve exists for safe insertion of the Watchman device. Among many other things, a physician who implants a Watchman anti-stroke device must:
- determine which of 5 device sizes is most appropriate for a particular patient
- determine which type of access sheath is most appropriate for the patient
- confirm that no blood clots currently exist in the patient’s left atrium and left atrial appendage
- take steps to avoid introducing air bubbles (emboli) during insertion
- take steps to recapture partially or fully implanted devices when not safely inserted
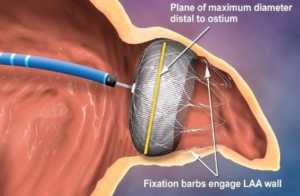
- embolization of the device during implantation (movement from the intended site that results in a blockage in the patient’s heart)
- breakage of the metal device during insertion
- air bubble in a vein or artery (air embolism)
- pericardial effusion (excess fluid around the heart)
Watchman device insertion complications can lead to life-threatening injuries or death. Both air emboli and device embolization can cause a patient’s stroke. Device breakage can puncture a patient’s heart or blood vessels, and pericardial effusion can reduce the function of a patient’s heart.
Watchman stroke devices can cause serious injuries during insertion as well as following insertion.